Abstract
Background
Lenalidomide, bortezomib, and dexamethasone (RVD) is a standard front-line regimen for both transplant eligible and ineligible multiple myeloma (MM) patients. Based on original APEX study data, obtaining a complete blood count (CBC) before every administration of bortezomib has been recommended. Waiting for labs could add several hours to each visit. This can be inconvenient and costly for patients, payers, and institutions. There is a clear need to decease the frequency of labs drawn to promote increased cost-savings, improved patient safety, and decreased clinic wait times.
Methods
The primary objective of this study was to implement and evaluate a process change to decrease the frequency of labs for eligible patients on RVD for MM. First, an institutional review board-approved descriptive, retrospective study that included patients aged ≥ 18 years with MM receiving treatment with RVD was performed to assess trends in lab values. The objective of the retrospective review was to identify patients who can safely receive RVD without repeat labs prior to each bortezomib injection. Results from this retrospective review was presented to Dana-Farber Cancer institute (DFCI) and Massachusetts General Hospital myeloma clinicians, as well as the DFCI Pharmacy and Therapeutics committee. After approval was granted to implement institutional changes, we evaluated the impact of the process change.
Results
Retrospective study results
Eighty-nine patients were included in the study. All patients had a platelet (PLT) count ≥ 75,000 cells/µL on day 1 of cycle 2 and beyond. Grade ≥ 3 thrombocytopenia developed in less than 3% of patients.
Greater than 93% patients had an absolute neutrophil count (ANC) ≥ 1,000 cells/µL on day 1 of cycle 2 and beyond. Grade 3 and 4 neutropenia developed in 6.7% and 1.1% of patients, respectively.
This study demonstrated that beyond the first cycle, patients with PLTs ≥ 75,000 cells/µL and an ANC ≥ 1,000 cells/µL on the first day of a cycle do not need labs prior to each administration of bortezomib in the cycle. These results provided the rationale for implementation of a new routine workflow.
Workflow Implementation
Implementation of a new workflow started on July 1, 2021 and included 71 adult patients receiving RVD for MM. First, a communication order was added to all RVD treatment plan templates in the electronic medical record specifying, "if on Day 1: ANC is ≥ 1,000 and PLTs are ≥ 75,000, no labs are required for remainder of that cycle." Following that, a flowchart was developed by nursing that instructed infusion nurses to request a cancelation of future labs within the current treatment cycle (if the patient met criteria).
Workflow Evaluation
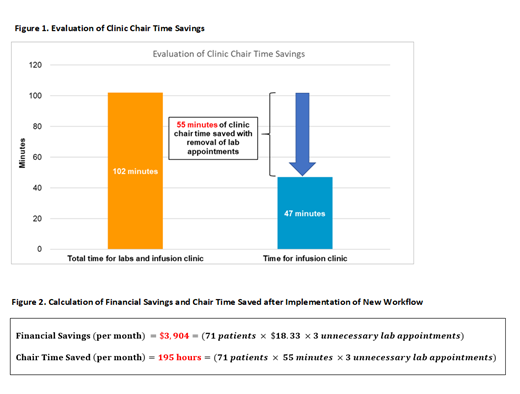
The Centers for Medicare & Medicaid Services reimbursement rates for a comprehensive metabolic panel and CBC with differential is $18.33. Following the implementation of this workflow, we saved over $3,904 in unnecessary healthcare related costs per month (figure 2).
The average time it takes for our patients on RVD to check into their lab appointment and have labs resulted is 55 minutes. It takes an average of 47 minutes for patients to have bortezomib administered after labs are reviewed and orders are released for preparation (figure 1). Ultimately, patients spend more than 50% of their time in clinic waiting for labs compared to the time it takes to have bortezomib prepared, delivered, and administered. The implementation of this new workflow resulted in saving 195 hours of clinic chair time per month (figure 2).
This time saved results in improved quality of life for patients who already have multiple visits at various healthcare facilities. Spending less time in clinic and reducing the frequency of venipunctures could potentially reduce the risk of bleeding, bruising, and discomfort amongst a patient population that is typically older and more at risk for complications.
Conclusion
This study demonstrates that it is economical, resourceful, and safe to implement a workflow process aimed at decreasing the frequency of lab draws in patients receiving RVD for multiple myeloma. It allowed our institution to maximize chair time that could be used for other patients and generate additional value-added revenue. This is particularly very important with the Covid-19 pandemic, where reducing several hours of wait time will keep our patients and staff safer.
Munshi: Legend: Consultancy; Janssen: Consultancy; Abbvie: Consultancy; Adaptive Biotechnology: Consultancy; Karyopharm: Consultancy; Oncopep: Consultancy, Current equity holder in publicly-traded company, Other: scientific founder, Patents & Royalties; Takeda: Consultancy; Amgen: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal